 | |
| Species: |
Rat |
| Strain/breeder: |
HanIbm : WIST (SPF) |
| Sex: |
Female |
| Age: |
9 Weeks |
| Study type: |
Repeat-Dose Neurotoxicity: Positive Control Study |
| Treatment: |
Neurotoxicant, 30 mg/kg, 15 daily doses; oral (by gavage) |
| Animal status: |
Scheduled sacrifice, end of study |
| Clinical findings: |
Abnormal gait during the 2nd week of the exposure period |
| Organ(s): |
CNS (Cerebellum) |
Macroscopic
finding(s): |
None |
| Staining: |
H&E |
|
 |
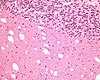
Fig. 1 (91k)
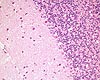
Fig. 2 (96k)
|
|